Personalized medicine aims to provide an exact treatment, for a unique individual, exactly when it is needed for the best possible health outcomes. To achieve this goal, healthcare needs longitudinal, large-scale, diverse clinical trials that truly represent the broad population.
Yet in analyzing all published randomized clinical trials in the New England Journal of Medicine, JAMA, and The Lancet between 2015 and 2019, only half the studies reported on the race of their participants. Amongst these studies, most of the participants were caucasian, accounting for 84% in 2015 and 77% 2019.
Without ethnic diversity, studies run the risk of generating data with blind spots for vast swaths of the population. Recent genome-wide association studies have found specific genetic variations between different ethnicities that may impact risk of disease. Genetic ancestry may also impact effectiveness of treatments, dosages required, and outcomes.
Gender diversity is equally important. Since most clinical trial participants are males, it’s no surprise that women have adverse drug reactions at almost twice the rate as men. Gender differences in dosages, effectiveness, and side effects of medicines are not often studied. And the consequences are being felt. Of all the prescription drug recalls between 1997 and 2001, 80% of them posed greater health risks for women than men.
This is why only through diversity in studies can we develop personalized medicine. Studying diverse demographics and combining statistical analysis with prognostic data is the only way to gauge if a treatment is effective across all populations.
The traditional clinical trial approach will inherently struggle to have diverse study populations. Virtual studies can reach individuals anywhere, and be run more affordably, enabling the scale and diversity in trials needed to advance towards personalized medicine.
Diverse Clinical Trials are Key for Personalized Medicine
Diversity is vital to personalized medicine, since patients of varying ages, races, and genders can metabolize compounds differently. However, diversity is not dependent upon genetics alone. In fact, geneticists publishing in the journal Nature recently called for developing “more holistic measures of individual risk” in large-scale clinical studies that are both genetic and non-genetic.
Holistic collection, evaluation, and comparison of data can find patterns in disease risk among races. For example, studies found that four times the number of African Americans suffer from kidney disease compared to white Americans. This led to healthcare recommendations adjusting thresholds for kidney disease screening for African Americans. While this knowledge is important, it is not holistic. It is only studying genes.
Holistic approaches would consider socioeconomic and lifestyle factors in addition to genetics, leading to precise care that could look for high blood pressure and diabetes, along with kidney disease in at-risk populations. The NIH stands behind this approach, noting the importance of diversity in research and clinical studies.
Even with ethnic and gender representation, diversity in clinical trials can still be missed if these holistic factors are not represented, leaving out entire populations. Intentional heterogeneity is not easy to attain, but studies are being done to lay framework approaches to achieving a truly diverse study.
Including all populations in all research will improve health outcomes for all.
Dr. Jeff Chen
Radicle Science
Barriers to Inclusivity in Traditional Clinical Studies
While the research community agrees on the importance of diversity, why has it been so hard to achieve in clinical trials? Most trials are localized and homogenized—clustered in urban areas—due to three barriers that are also the hardest to overcome:
- Trial site location
- Time commitment
- Discomfort in healthcare settings
Morning Consult conducted a poll that found most Americans stated ease of access is important for participating in trials. Diversity in clinical trials was equally as important.
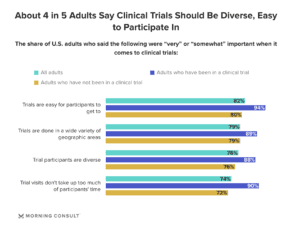
Overcoming location restrictions can be achieved through satellite clinics or at-home visits, but they introduce another limiting factor—cost, which can often be more than a traditional site-based approach. Fully virtual studies open up participation without the burden of added cost and allow for tremendous scalability.
Overcoming Barriers Through Virtual Studies
Virtual trials overcome barriers of distance and transportation access to trial sites, while simultaneously reducing time commitment. A rural African American farmer and a minimum wage earning, Latin-X single parent face the same challenge in participating in clinical trials.
Traditional trials take place during weekday hours, when participants must balance lost wages, lost childcare, and lost time with participating. Virtual trials minimize time commitment and allow for greater diversity across socioeconomic lines.
Virtual studies were the most popular option amongst polled Americans interested in participating in clinical trials.
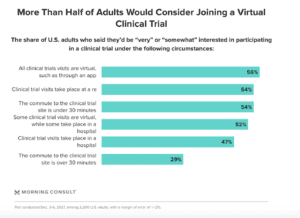
Virtual studies overcome most geographic and socioeconomic barriers and avoid the discomfort of healthcare facilities, which is especially pertinent for minority populations who historically have distrusted the system.
This is why Radicle Science pioneered our virtual, crowdsourced, direct-to-consumer revolutionary approach to diverse clinical trials. By allowing participants to participate in research from the comfort of home, we are achieving diversity across gender, ethnicity, geography, socioeconomic status, and lifestyle – all in our mission to bring us closer to personalized medicine for all.
Turning Diverse Clinical Trial Results Into Personalized Medicine
Truly representative data is the key in achieving personalized medicine, yet aggregating the data and accounting for every possible variable in all outcomes remains a challenge. To bridge this gap, we need standardized approaches to data content, formatting, and clinical definitions.
Common assessment and protocols are the first step in creating universal methodologies to provide personalized medicine, which can be achieved by:
- Aggregating and anonymizing all data sets
- Researching hidden correlations between all demographic variables
- Reviewing predictive insights
This is why Radicle Science has set a new standard with our standardized study protocols. Not only does our standardization allow for deep AI analysis across big data sets, but it also eliminates any biases that can be present in custom-designed study protocol. We are conducting third-party gold standard double-blind randomized placebo controlled clinical research that is the gold standard in objectivity – all at unprecedented scale, speed, and affordability.
Personalized medicine is at our doorstep. As each large-scale diverse clinical trial takes place, we are growing ever closer to personalized medicines for all peoples.
Learn more about how your brand can be part of diverse clinical trials and subscribe to our newsletter below!
Interested in volunteering to be a participant in our supplement clinical trials? Subscribe below to be the first to know of open studies!


